Build Your Regenerative Medicine Authority Regenerative medicine authority starts with smart plans. Plus, you need strong leaders. Also, you must help patients. Therefore, this takes real work. The field needs great leaders today. Moreover, these leaders mix science with business. Thus, they help change patient care. Meanwhile, data proves the methods work. In fact, they
Miami, Florida – Global Stem Cells Group (GSCG) is thrilled to announce the grand opening of its latest state-of-the-art Stem Cell Center, located in Jakarta, Indonesia. This momentous occasion marks a significant stride forward in the field of regenerative medicine and showcases GSCG’s commitment to providing innovative healthcare solutions worldwide. In a groundbreaking partnership with
Miami, Florida – Global Stem Cells Group, a leading organization in the field of regenerative medicine, is pleased to announce the appointment of Dr. Salih Yildirim as the new President of the International Society for Stem Cell Application (ISSCA), a division of Global Stem Cells Group. Dr. Yildirim previously held the position of Director of
Miami, Florida – The International Society for Stem Cell Application (ISSCA), a division of Global Stem Cells Group (GSCG), successfully launched its inaugural training course in Hormonal Modulation and Gynecology, marking a significant milestone in the field of regenerative medicine. The course, held from June 10-12, 2023, in Cancun, Mexico, generated immense enthusiasm among participants
Collaboration Set to Propel Regenerative Medicine Research and Innovation Miami, May 20, 2023 – Global Stem Cells Group, a leading provider of regenerative medicine solutions, is thrilled to announce the opening of its state-of-the-art research facility at Marmara University’s Main Campus in Istanbul, Turkey. This collaborative endeavor between Global Stem Cells Group, ReGen (described below),
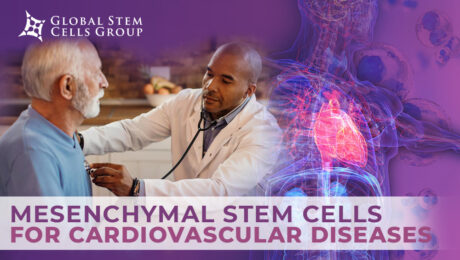
Despite progress in cardiovascular research, cardiac pathology continues to be one of the most common causes of morbidity and mortality in the world. Stem cell-based therapy has been recognized as an innovative strategy for the repair, regeneration and functional recovery of the myocardium, hence, once the animal research stage has been overcome, most clinical trials
Atherosclerosis is the most common form of arterial occlusive disease in adults. About 15 percent of adults over 55 years of age suffer from critical ischemia, the most severe form of this disease. Due to the gradual aging of the population and the growing number of people in their third age group, a number of
In recent years, MSCs have been introduced as respectable candidates for regenerative medicine due to their pro-angiogenic, anti-apoptotic, and immunomodulatory attributes. A variety of human tissues can be used as a source of mesenchymal stem/stromal cells (MSCs), ranging from bone marrow (BM) to umbilical cord (UC). These cells are typically multipotent and can differentiate into
Miami, FL, December 12, 2022 – Global Stem Cells Group (GSCG) is pleased to announce the opening of a new Stem Cell Center in Bandung, Indonesia in partnership with the Dr. Yanti Aesthetic Clinic. This joint venture will be the second in the country as they already have another facility in the stunning City of