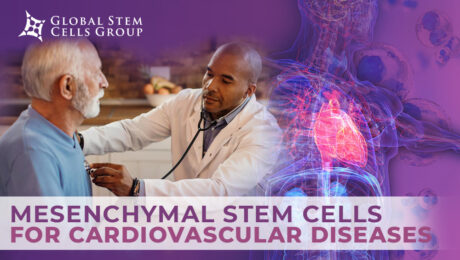
Despite progress in cardiovascular research, cardiac pathology continues to be one of the most common causes of morbidity and mortality in the world. Stem cell-based therapy has been recognized as an innovative strategy for the repair, regeneration and functional recovery of the myocardium, hence, once the animal research stage has been overcome, most clinical trials
Atherosclerosis is the most common form of arterial occlusive disease in adults. About 15 percent of adults over 55 years of age suffer from critical ischemia, the most severe form of this disease. Due to the gradual aging of the population and the growing number of people in their third age group, a number of
In recent years, MSCs have been introduced as respectable candidates for regenerative medicine due to their pro-angiogenic, anti-apoptotic, and immunomodulatory attributes. A variety of human tissues can be used as a source of mesenchymal stem/stromal cells (MSCs), ranging from bone marrow (BM) to umbilical cord (UC). These cells are typically multipotent and can differentiate into
Miami, FL, December 12, 2022 – Global Stem Cells Group (GSCG) is pleased to announce the opening of a new Stem Cell Center in Bandung, Indonesia in partnership with the Dr. Yanti Aesthetic Clinic. This joint venture will be the second in the country as they already have another facility in the stunning City of
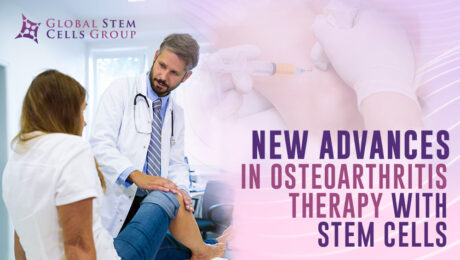
Osteoarthritis is a rheumatic pathology that damages the articular cartilage. By joining two bones through the joint capsule, the joints are able to move, providing us with functional autonomy. An inner fluid called synovial fluid is usually found within joints, which is produced by the synovial membrane. Articular cartilage covers the ends of the bones
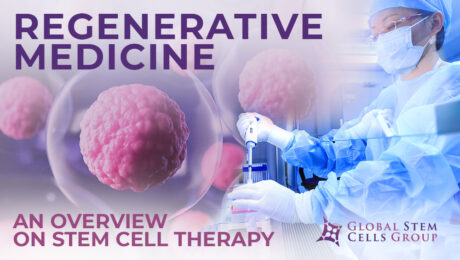
Stem cell therapy is a form of regenerative medicine designed to repair damaged cells within the body by reducing inflammation and modulating the immune system. This phenomenon makes stem cell therapy a viable treatment option for a variety of medical conditions. What is stem cell therapy? The term stem cell therapy refers to any treatment
Global Stem Cells Group, Announces Launch of New Stem Cells and Regenerative Medicine Clinic in CDMX
Miami, FL, October 28, 2022 – Global Stem Cells Group announces a new partnership that enhances its goal of establishing its therapies and technology to meet market demand in populated areas of the world. This collaboration with STEM LIFE clinic’s new facility and Dr. Vanessa Rodriguez Pares, currently one of the most prestigious aesthetic clinics in Mexico
Global Stem Cells Group has just announced the official opening of a new multi-specialty regenerative medicine center in Cancun. The new center is intended to incorporate different treatments based on regenerative medicine as well as to serve as a multi-specialty training center in the field of regenerative medicine and cellular therapies, offering doctors and patients
Global Stem Cells Group (GSCG) has announced the appointment of Dr Rafael Moguel as the new Chief Medical Officer (CMO) of Cellular Hope Institute Cancun. Dr. Moguel, Fellow of the Society for Cardiac Angiography and Intervention (SCAI), Member of the Mexican Society for Interventional Cardiologists, and Member of the Latin American Society for Interventional Cardiology,
The International Society of Stem Cell Applications (ISSCA) held its first World Congress on Regenerative Medicine at the Radisson Blu Hotel in Istanbul, Turkey between the 23rd and 26th of September, 2022. Like the previous events, the Congress brought together world-class physicians and scientists from over 35 countries, giving medical professionals access to first-rate live