
One major aims of regenerative medicine is to replace lost tissue with new cellular material or to improve the regeneration of damaged, malfunctioning, diseases tissue and organs using stem cell transplantation. In view of this, the discovery of Extracellular vesicles (EVs) in the twentieth century have being considered as significant factors in inflammation and immune responses, antigen presentation, immunomodulation, coagulation, tissue regeneration, organ repair, cell-cell communication, senescence, proliferation and differentiation in the body [1]. Extracellular vesicles are believed to be involved in many biological processes and they can be modified to contain specific proteins, genetic lipids, and genetic materials including messenger RNA (mRNA), microRNA (mRNA), and other small non-coding RNAs, and genomic DNA (gDNA) from their progenitor cell.
Extracellular vesicles are classified into two groups which include; exosomes and ectosomes [2]. Exosomes are characterized with cup-shaped morphology, appearred as flattened spheres with diameters ranging from 30 to 150 nm. Similarly, exosomes have a characteristic lipid bilayer which has an average thickness of ∼5 nm. [3]. Thus, the lipid components of exosomes include ceramide (sometimes used to differentiate exosomes from lysosomes), cholesterol, sphingolipids, and phosphoglycerides with long and saturated fatty-acyl chains. The exosome is formed or derived from a multivesicular body (MVB) when the MVB fuses with the plasma membrane and is released into the extracellular environment.
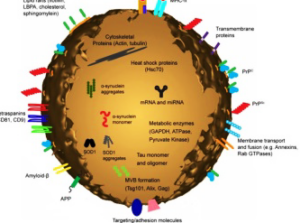
Diagrammatic representation of a medium size Exosome.
Exosomes contain many types of biomolecule, including proteins, carbohydrates, lipids and nucleic acids which vary depending on the EV’s origin, its physiological and pathological state, and even the precise cellular release site. Thus, the protein composition within can also mark the existence of disease pathologies such as inflammatory diseases; however, exosomes also contain a number of common proteins as well as those that participate in vesicle formation and trafficking. Furthernore, exosome plays a role in intercellular communication by carrying proteins and RNAs between neighboring cells or even to distant organs, they also bind to cell membranes through receptor– ligand interaction and mediate antigen presentation, cancer progression etc.
Various techniques have being developed for the isolation of exosomes which includes; ultracentrifugation-based isolation techniques, size-based techniques, precipitation techniques, immune-affinity capture-based techniques, and some novel combination techniques [4]. Exosomes primarily exist in pellets after centrifugation 100000–200000×g and the use of Ultracentrifugation and ultrafiltration can be used to obtain purified exosomes in the laboratory, but this technology is difficult to apply on a large scale [5].
Exosomes can be stored at 4°C for up to 1 week for short-term while it can be stored at -80°C for long-term by suspending it in phosphate buffered saline.
More importantly, growing evidence has also suggested that exosomes play a key role in facilitating tumorigenesis by regulating angiogenesis, immunity, and metastasis. Circulating exosomes in body fluids and blood in particular are potentially non-invasive or minimally invasive biomarkers for early diagnosis and prognosis of various types of cancer.[6] As the first step towards improving human health, exosomes have to be reliably and efficiently isolated from complex biological matrices like blood, urine, and cerebrospinal fluid since they are currently tested as next-generation biomarkers in those body fluids.
In conclusion, it is advantageous to use exosomes for cell based treatments because the use of exosomes can avoid problems associated with the transfer of cells, which may already have damaged or mutated DNA [7]. Also, exosomes are small and can easily circulate through capillaries, whereas the cells used in other cell-based therapies, such as MSCs, are too large to go through capillaries, and thus cannot get beyond first pass capillary beds, such as the lungs.
The level of MSCs in cell-based therapies may quickly diminish after transplant while, exosomes can achieve a higher “dose” than the transplanted MSCs [8]. Similarly, exosomes can also be utilized to tackle toxicity and immunogenicity problems resulting from such biomaterial treatments as nanoparticles [9].
CLINICAL APPLICATION
Ø Immuno-modulatory and anti-inflammatory properties of Exosomes could be the potential biological mechanisms for clinical treatment to promote bone regeneration. [10]
Ø Adipose-derived stem cell-derived exosomes promote fracture healing in animals by binding to polylactic acid-glycolic acid scaffolds.
Ø Exosome can be used for the treatment of chronic kidney disease, type 1 diabetes mellitus, and skin damage [11].
Ø MSCs-derived exosomes have shown therapeutical benefits in stroke and intravenous administration of MSCs-derived exosomes induced an increase of neurogenesis, neurite remodeling, and angiogenesis. [12]
Ø Administration of MSCs-derived exosomes’ has being observed in a traumatic brain injury model by showing an inflammation reduction and good outcomes.[13]
Ø The injection of MSCs-derived exosomes has also been shown to be a possible treatment for spinal cord injury (SCI), by reducing inflammation and by promoting neuro-regeneration in rats after injury [14].
Ø Exosomes can be used as a delivery system of therapeutic signals or drugs due to their low immunogenicity, ability to cross the blood-brain barrier (BBB), and long half-life in circulation.



